CBSE Class 11 Chemistry Notes Chapter 8 Redox Reactions

CBSE Class 11 Chemistry Notes Chapter 8: Redox Reactions focus on helping students clearly understand the traditional concept of oxidation and reduction. These notes explain how redox reactions take place through the transfer of electrons, change in oxidation numbers, and involvement of oxidising and reducing agents. The main aim of class 11 chemistry chapter 8 notes is to make difficult concepts easy to learn and remember.
The class 11th redox reaction notes start with the basic meaning of oxidation and reduction. Students learn that oxidation is the loss of electrons, while reduction is the gain of electrons. The chapter also covers important topics such as oxidation number rules, balancing redox reactions, and electrode processes. These concepts are explained step by step in chemistry notes for class 11 chapter 8, making them useful for both classroom learning and exam revision.
Using redox reaction short notes, students can quickly revise key formulas, definitions, and examples before tests. These notes also include real-life examples to help students connect theory with practical applications. This makes learning more interesting and effective.
The class 11th chemistry chapter 8 notes are designed to save time and improve understanding. Students can use these notes to rewrite answers in their own words and strengthen their basics. The chemistry class 11 redox reactions notes are especially helpful for board exams, as questions from this chapter are often concept-based.
Check Out: Class 11th Books
CBSE Class 11 Chemistry Notes Chapter 8
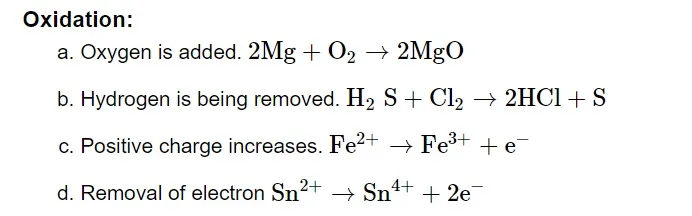
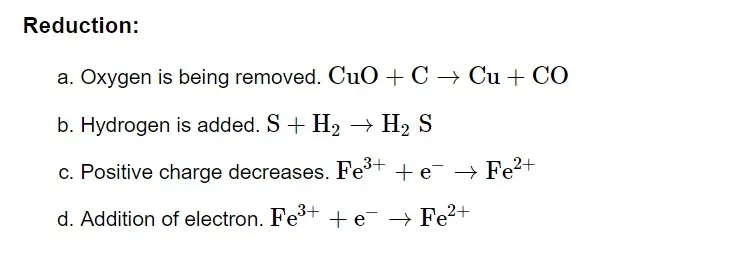
Oxidation Number
An element acquires an imaginary or appearing charge over each atom when it changes from its elemental free state to its combined form in molecules. It is ascertained by applying a random set of guidelines. In a specific bonded state, the charge is relative. It has proven possible to use oxidation numbers as a more useful method to monitor electron changes in chemical reactions involving compound formation. Throughout this process, the full transfer of electrons from a less electronegative to a more electronegative atom is always anticipated.
Rules Governing Oxidation Number
The oxidation number of each element in a variety of compounds can be determined using the following formulas. It's crucial to keep in mind that these guidelines are based on the element's electronegativity.
Atom of Fluorine
Fluorine is the most electronegative of all the elements (known). In all of its compounds, it has an oxidation number of –1.

Hydrogen Atom
The hydrogen atom has an oxidation number of +1 in general. However, it is –1 in metallic hydrides (e.g. NaH, KH).
Halogen Atom
In general, all halogen atoms (Cl, Br, and I) have an oxidation number of –1.
If a halogen atom is connected to a more electronegative atom than the halogen atom, the oxidation numbers will be positive.
Metals
Alkali metals (Li, Na, K, Rb, etc.) always have an oxidation number of +1. For alkaline earth metals (Be, Mg, Ca, etc.), the oxidation number is always +2. Aluminum's oxidation number is always +3. Keep in mind that a metal's oxidation number could be zero or negative. The oxidation number of an element is always 0 when it is in the free state or allotropic forms. In a molecule, the total oxidation number of all the atoms is zero. An ion's charge is determined by adding the oxidation numbers of each of its constituent atoms. In the modern periodic table, an element with group number n can have an oxidation number between (n – 10) and (n – 18). (However, it mostly applies to p-block elements).
Paradox of Fractional Oxidation Number
The fractional oxidation number is the average of the oxidation states of all the atoms of the element under consideration, and the structural characteristics show that the atoms of the element for which the fractional oxidation state is realized are indeed present in separate oxidation states.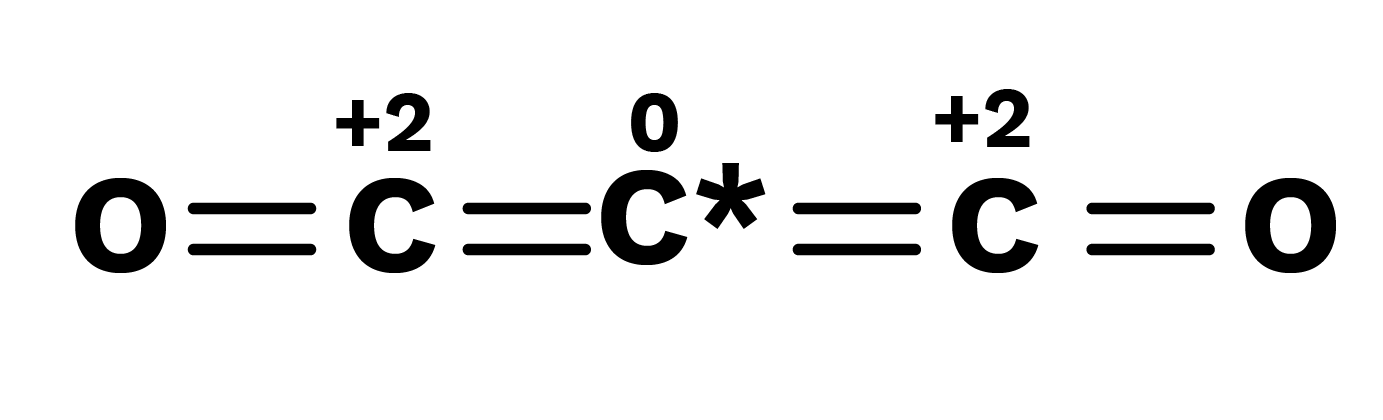
Oxidizing and Reducing Agent
Oxidising Agent or Oxidant
Oxidizing agents are compounds that, in the course of a chemical reaction, can both oxidize and reduce other molecules. Chemicals known as oxidants can change an element's oxidation number in a redox process by adding or subtracting electrons.
Reducing Agent or Reductant
Reducing agents are compounds that, in a chemical reaction, can both reduce and oxidise other molecules. Reductants are substances that raise an element's oxidation number or cause it to lose electrons during a redox reaction.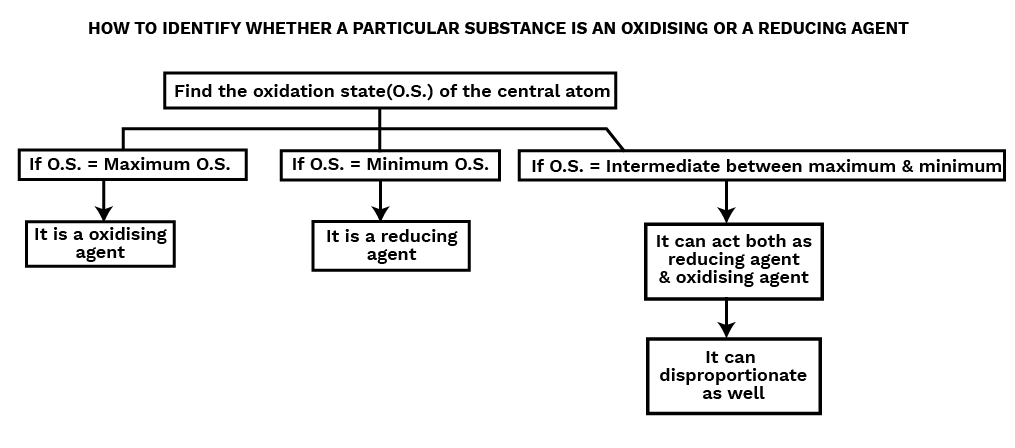
Disproportionation Reaction
A disproportionation reaction is a type of redox reaction in which an element that is present in a particular molecule at a certain oxidation state is simultaneously reduced and oxidised. Disproportionation reactions are a special kind of redox reaction. An element that can exist in at least three oxidation states must always be present in one of the reactants in a disproportionation process.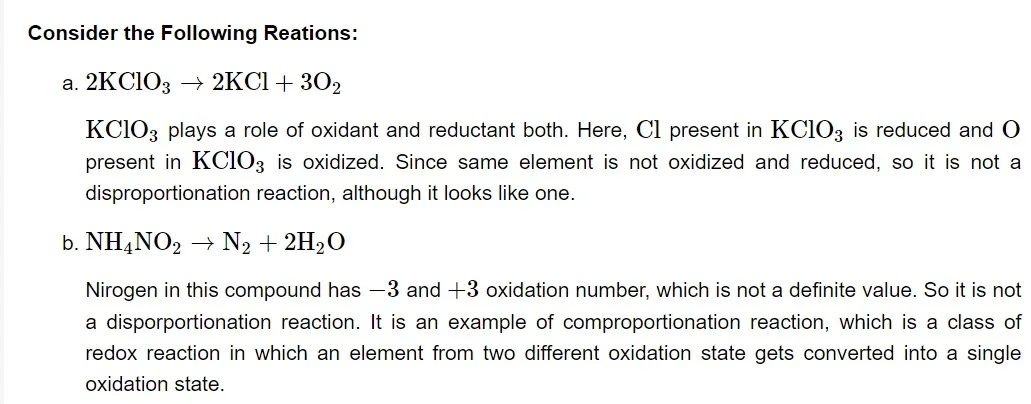
Balancing of Redox Reactions
Two conditions must be met by any balanced equation. Atom balance, also known as mass balance, states that there should be an equal amount of atoms of each species on the reactant and product sides. Charge balance: The total of the real charges on both sides of the equation ought to be the same.
The redox equations can be balanced in two ways:
-
Number change technique of oxidation
-
Half-cell method or ion electron method
Because the first approach is not particularly effective in balancing redox reactions, students are urged to use the second way (Ion electron method) to do so.
Acid-Base Reaction
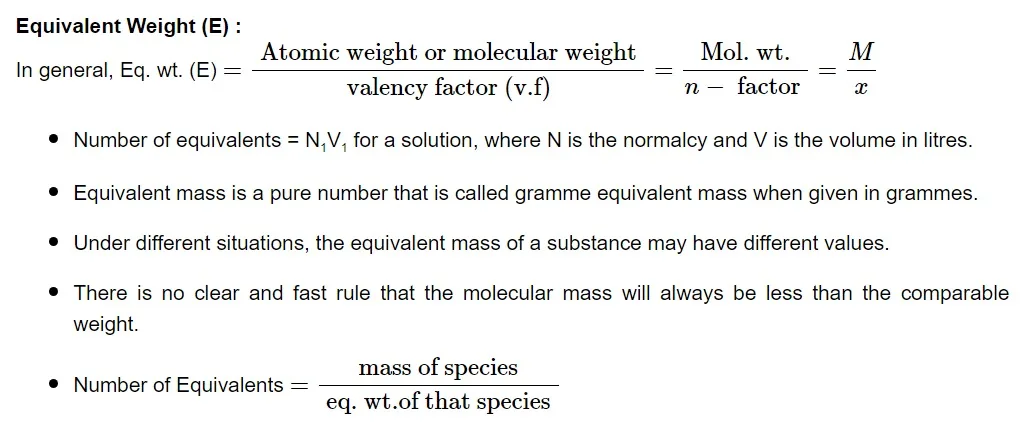
The actual number of hydrogen or hydroxide ions transferred during an acid-base reaction is known as the valence factor. The acid or base may contain more hydrogen or hydroxide ions that can be replaced than what is replaced throughout the reaction. V. f is the number of hydrogen ions from the acid that are substituted for each base molecule.
Normality
The normalcy of a solution is defined as the number of equivalents of solute in one liter (1000 mL). Allow W g of the equivalent weight E solute to dissolve in water to produce V mL of solution.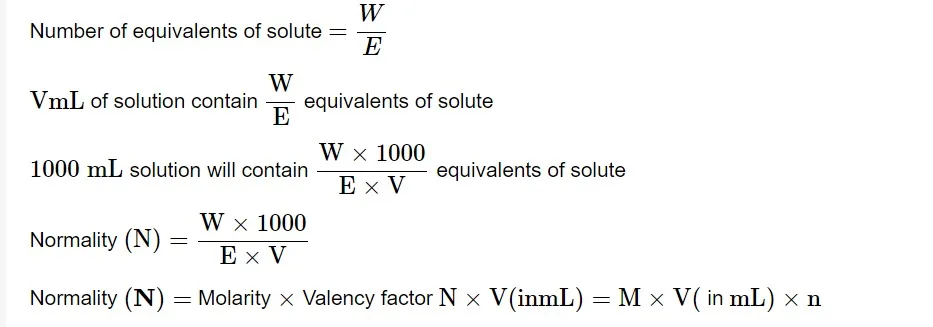
Law of Equivalence
The law states that one equivalent of one element combines with one equivalent of the other. A chemical reaction produces the same number of equivalents or milli equivalents of products separately from equivalents and milliequivalents of reactants in the same proportion.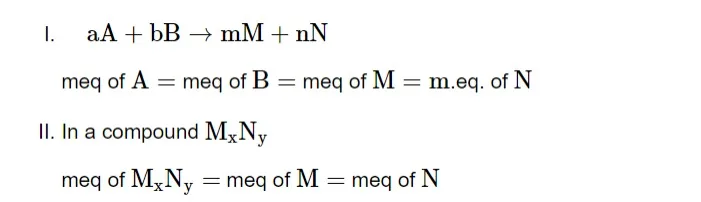
Titrations
By allowing a precisely measured volume to react with a reference solution of a different substance that has a known concentration, titration is a technique for measuring the concentration of a solution. A known concentration solution taken in a burette is called a standard solution. Another name for it is titirant.
There are Two Types of Titrants
Primary Titrants/Standards - These reagents can be precisely weighed, and their solutions do not require standardisation before use. Example: oxalic acid, ferrous ammonium sulfate.
Secondary Titrants/Standards: Because these reagents cannot be precisely weighed, their solutions must be standardised before use. Example: NaOH,KOH NaOH,KOH
Titrate: A solution containing the chemical to be measured, usually in a beaker.
The equivalency point is the point at which the number of titrant equivalents added equals the number of titrate equivalents.
Indicators
extra material supplied to enable the titration completion at the equivalency point to be physically detected. Usually, the hue changes when the titration is complete. There are numerous sizes and shapes for titrations.
Types of Indicators:
-
Titrations of acid and base (to be studied in Ionic equilibrium)
-
Permanganate titration:
Check Out: Class 11th Question Banks
How to Use CBSE Class 11 Chemistry Chapter 8 Notes
Start with the NCERT Textbook
Before using the notes, carefully read the full Chapter 8 Redox Reactions from the NCERT Chemistry textbook. This will help you understand the basic ideas of oxidation, reduction, oxidation number, and electron transfer. While reading, pay attention to definitions, examples, and reaction equations.
Learn Each Concept from the Notes
After reading the textbook, go through the CBSE Class 11 Chemistry Chapter 8 notes topic by topic. These notes explain redox reactions, balancing equations, and electrode processes in simple words. Study one concept at a time and relate it to the examples given in the NCERT book.
Revise Key Points Regularly
Use the notes for regular revision of important formulas, rules for oxidation numbers, and key definitions. Frequent revision will help you remember concepts easily during exams.
Practice Numerical and Theory Questions
Once you complete the notes, practice numerical problems and theory questions from the textbook and sample papers. The notes will help you understand the steps and write correct answers.
Add Extra Examples and Tips
If your teacher explains extra shortcuts or gives important exam tips, add them to your Class 11 Redox Reactions notes. This will make your notes more useful and exam-ready.
Read More: CBSE Sample Paper Class 11 with Solutions PDF
CBSE Class 11 Chemistry Notes Chapter 8 FAQs
Q1. What are redox reactions in Class 11 Chemistry?
Redox reactions are chemical reactions in which oxidation and reduction occur together. Oxidation means loss of electrons, while reduction means gain of electrons.
Q2. Why are Class 11 Redox Reaction notes important for exams?
Class 11 redox reaction notes explain concepts like oxidation number, electron transfer, and balancing reactions in a simple way, which helps in quick revision and better exam performance.
Q3. What topics are covered in Class 11 Chemistry Chapter 8 notes?
The chapter covers oxidation and reduction, redox reactions in terms of electrons, oxidation numbers, balancing redox equations, and electrode processes.
Q4. How can redox reaction short notes help students?
Redox reaction short notes help students revise key definitions, formulas, and examples quickly before tests and exams.
Q5. Are Class 11 Chemistry Chapter 8 notes useful for board exams?
Yes, Class 11 Chemistry Chapter 8 notes are very useful for board exams as many questions are concept-based and directly come from redox reactions.










