NCERT Solutions for Class 9 Science Chapter 4 Structure of The Atom
Knowing the structure of an atom is like unlocking the universe's secrets! For Class 9 students, Chapter 4, Structure of the Atom, is a foundational topic that explains how everything around us is made of tiny particles called atoms. We will summarize the chapter’s key concepts and provide simple NCERT solutions to common questions. This is really an interesting chapter and a practical theory that lets you know what every living and non-living thing is made of.
Check Out: Class 9th Books
NCERT Solutions for Class 9 Science Chapter 4
You can find the NCERT Solutions for Class 9 Science Chapter 4 "Structure of the Atom" below. It is a valuable resource for students looking to deepen their understanding of chemistry and physics concepts.
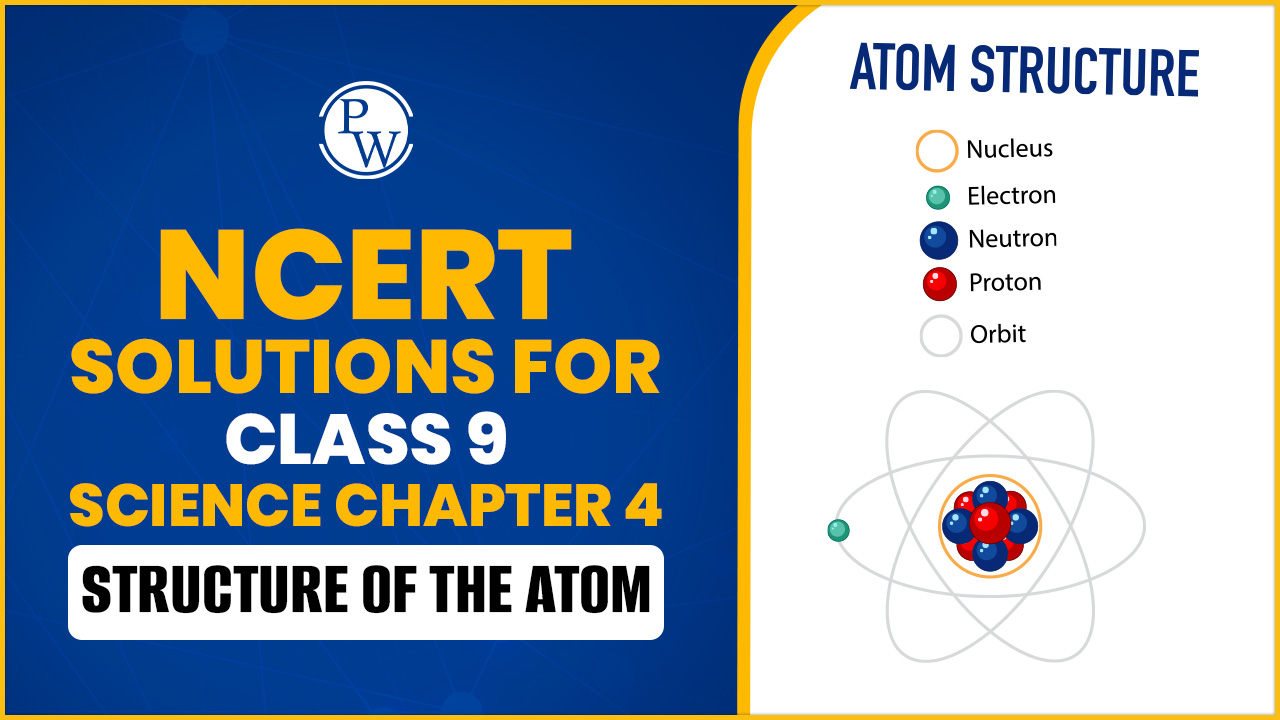
Structure of the Atom
The structure of an atom refers to its fundamental composition and organization. Atoms are the basic building blocks of matter and consist of three main subatomic particles:
Protons: Positively charged particles found in the nucleus of the atom. Each proton has a relative mass of approximately 1 atomic mass unit (amu).
Neutrons: Neutrally charged particles also located in the nucleus. Neutrons have a mass similar to protons, approximately 1 amu, but they do not carry an electrical charge.
Electrons: Negatively charged particles that orbit the nucleus in specific energy levels or shells. Electrons have negligible mass compared to protons and neutrons.
Read More: NCERT Solutions for Class 9 Science Chapter 1
Class 9 Science Chapter 4 Structure of the Atom Question Answer
Below, we have provided NCERT Solutions for Class 9 Science Chapter 4: Structure of the Atom for the students' convenience.
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom Exercise-4.1 Page: 47
1. What are canal rays?
Solution: The radiations that are positively charged are canal rays. This discovery was crucial in the discovery of another subatomic particle that was positively charged – the proton.
2. If an atom contains one electron and one proton, will it carry any charge or not?
Solution: Since a proton is a positively charged particle and an electron is a negatively charged particle, the net charge becomes neutral as both particles neutralise each other.
Read More: NCERT Solutions for Class 9 Science Chapter 2
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom Exercise-4.2 Page: 49
1. On the basis of Thompson’s model of an atom, explain how the atom is neutral as a whole.
Solution: Thomson's model of the atom, proposed in the late 19th century, suggested the following: (i) An atom is envisioned as a uniform sphere filled with positive charge, with negatively charged electrons embedded within it like raisins in a pudding. (ii) According to Thomson's model, electrons and the positive charge are distributed uniformly throughout the atom, making the atom electrically neutral overall. This concept of equal positive and negative charges within the atom led to it being referred to as the "plum pudding model."
2. On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Solution: Rutherford's model of the atom, proposed around the early 20th century, introduced significant changes from Thomson's model:
In Rutherford's model:
-
The atom is mostly empty space, with a tiny, dense, positively charged nucleus at its center.
-
This nucleus contains positively charged protons and nearly all of the atom's mass.
-
Electrons, which are negatively charged, orbit the nucleus at a distance.
Rutherford's experiments, particularly the gold foil experiment, led to the discovery of the atomic nucleus and the understanding that most of an atom's mass and positive charge is concentrated in this small nucleus.
3. Draw a sketch of Bohr’s model of an atom with three shells.
Solution:
4. What do you think would be the observation if the ∝– particle scattering experiment is carried out using a foil of a metal other than gold?
Solution: In the alpha particle scattering experiment, if any other metal foil is used instead of gold, the observations would generally remain consistent. This is because the fundamental structure of atoms, regardless of the element used, involves a dense nucleus surrounded by electrons in orbits or energy levels. Rutherford's experiment demonstrated that alpha particles, when directed at a thin foil of gold, sometimes bounced back or were deflected at large angles, indicating a concentrated positive charge (the nucleus) in the center of the atom. This basic atomic structure applies universally to all elements: a positively charged nucleus containing protons and neutrons, with electrons orbiting around it. Therefore, while the degree of scattering may vary depending on the atomic number and density of the foil used, the overall observation regarding the atom's structure would remain consistent across different metals.
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom Exercise-4.2.4 Page: 49
1. Name the three subatomic particles of an atom.
Solution: An atom consists of three subatomic particles:
-
Protons – Positively charged
-
Electrons – Negatively charged
-
Neutrons – Neutral in nature (no charge)
2. Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Solution: Given: Atomic mass of helium atom = 4u, 2 protons in helium nucleus Atomic mass = number of protons + number of neutrons 4 = 2 + number of neutrons Number of neutrons = 4 – 2 = 2 Hence, Helium has 2 neutrons.
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom Exercise-4.3 Page: 50
1. Write the distribution of electrons in Carbon and Sodium atoms.
Solution: The electron distribution in atoms describes how electrons are arranged in different energy levels or shells around the nucleus. Let's write the electron distributions given for carbon and sodium atoms:
Carbon Atom (Atomic Number 6):
-
1st shell (K-shell): 2 electrons
-
2nd shell (L-shell): 4 electrons
Electron distribution notation: 2, 4
Sodium Atom (Atomic Number 11):
-
1st shell (K-shell): 2 electrons
-
2nd shell (L-shell): 8 electrons
-
3rd shell (M-shell): 1 electron
Electron distribution notation: 2, 8, 1 These notations (2, 4 for carbon and 2, 8, 1 for sodium) indicate the number of electrons in each successive energy level or shell around the atom's nucleus. Each shell can only hold a specific number of electrons based on its energy level, following rules derived from quantum mechanics and atomic theory.
2. If the K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Solution: K shell can hold 2 electrons. L shell can hold 8 electrons. Hence, when both the shells are full, the total number of electrons present in the atom = 2+8 = 10 electrons.
Must Buy, Class 9th Sample Papers
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom Exercise-4.4 Page: 52
1. How will you find the valency of chlorine, sulphur and magnesium?
Solution: The Valency of an element refers to its tendency to gain, lose, or share electrons to achieve a stable electronic configuration, typically with a full outer shell. Mathematically, the valency can be determined based on the number of electrons in the outermost shell (also known as the valence shell):
-
If the outermost shell contains 4 or fewer electrons, the valency is equal to the number of electrons in that shell.
-
If the outermost shell contains more than 4 electrons, the valency is calculated by subtracting the number of electrons in the outermost shell from 8.
Calculation of valency:
Valency of chlorine:
The electronic configuration of chlorine = 2, 8, 7 Chlorine has 7 (more than 4) electrons in its outermost shell. Therefore, the valency of chlorine = 8 – the number of electrons in the outermost shell = 8−7 = 1 Valency of Sulphur: The electronic configuration of Sulphur = 2, 8,6 Sulphur has 6 (more than 4) electrons in its outermost shell. Therefore, the valency of chlorine = 8 – the number of electrons in the outermost shell = 8−6 = 2 Valency of magnesium: The electronic configuration of Magnesium = 2, 8, 2 Magnesium has 2 (less than 4) electrons in its outermost shell. Therefore, the valency of magnesium Number of electrons in its outermost shell = 2
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom Exercise-4.5 Page: 52
1. If the number of electrons in an atom is 8 and the number of protons is also 8, then
(i) What is the atomic number of the atom? and
(ii) What is the charge on the atom?
Solution: Given: Number of electrons = 8 Number of protons = 8 (i) The atomic number of an atom is the same as the number of protons in that atom; hence, its atomic number is 8. (ii) In an atom, the number of protons is equal to the number of electrons. Hence, both the charges – positive and negative – neutralise each other. Therefore, the atom does not possess any charge.
2. With the help of the given table, find out the mass number of oxygen and sulphur atom.
Table: Composition of Atoms of the First Eighteen Elements with Electron Distribution in Various Shells.
|
Name of Element |
Symbol |
Atomic number |
Number of Protons |
Number of Neutrons |
Number of electrons |
Distribution of electrons K L M N |
Valency |
|||
|
Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium |
H He Li Be B C N O F Ne Na Mg |
1 2 3 4 5 6 7 8 9 10 11 12 |
1 2 3 4 5 6 7 8 9 10 11 12 |
– 2 4 5 6 6 7 8 10 10 12 12 |
1 2 3 4 5 6 7 8 9 10 11 12 |
1 2 2 2 2 2 2 2 2 2 2 2 |
– – 1 2 3 4 5 6 7 8 8 8 |
– – – – – – – – – – 1 2 |
– – – – – – – – – — – – |
1 0 1 2 3 4 3 2 1 0 1 2 |
|
Aluminium Silicon Phosphorus Sulphur Chlorine Argon |
Al Si P S Cl Ar |
13 14 15 16 17 18 |
13 14 15 16 17 18 |
14 14 16 16 18 22 |
13 14 15 16 17 18 |
2 2 2 2 2 2 |
8 8 8 8 8 8 |
3 4 5 6 7 8 |
– – – – – |
3 4 3,5 2 1 0 |
Solution: (a) To find the mass number of Oxygen, Number of protons = 8 Number of neutrons = 8 Atomic number = 8 Atomic mass number = Number of protons + number of neutrons = 8 + 8 = 16 Therefore, the mass number of oxygen = 16 (b) To find the mass number of Sulphur, Number of protons = 16 Number of neutrons = 16 Atomic number = 16 Atomic mass number = Number of protons + number of neutrons = 16 + 16 = 32
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom Exercise-4.6 Page: 53
1. For the symbols H, D, and T, tabulate three subatomic particles found in each of them.
Solution: The following table depicts the subatomic particles in Hydrogen (H), Deuterium (D), and Tritium(T).
|
Isotope |
Symbol |
Mass no. |
Atomic no. |
No. of electrons |
No. of protons |
No. of neutrons |
|
Hydrogen |
H |
1 |
1 |
1 |
1 |
0 |
|
Deuterium |
D |
2 |
1 |
1 |
1 |
1 |
|
Tritium |
T |
3 |
1 |
1 |
1 |
2 |
2. Write the electronic configuration of any one pair of isotopes and isobar.
Solution:
(a) Isotopes: Isotopes are atoms of the same element that have the same number of protons (and therefore the same atomic number) but differ in the number of neutrons. This difference in neutrons results in isotopes having different mass numbers. Isotopes of an element behave similarly in chemical reactions because they have the same number of electrons and hence the same electronic configuration.
Example: Carbon has two major isotopes, Carbon-12 (6C12) and Carbon-14 (6C14). Both isotopes have the same atomic number (6) and thus the same number of protons and electrons, but Carbon-12 has 6 neutrons and Carbon-14 has 8 neutrons, leading to different mass numbers (12 and 14, respectively).
(b) Isobars: Isobars are atoms of different elements that have the same mass number but different atomic numbers. Unlike isotopes, which are of the same element, isobars are different elements altogether. This means isobars have different numbers of protons and electrons and therefore different atomic numbers.
Example: Calcium-40 (20Ca40) and Argon-40 (18Ar40) are isobars because they both have a mass number of 40. Calcium-40 has an atomic number of 20 (20 protons), while Argon-40 has an atomic number of 18 (18 protons). Despite the different atomic numbers, they can have similar electron configurations in their respective shells due to having the same number of electrons (20 for Calcium-40 and 18 for Argon-40) in their neutral state.
Check Out: Class 9th Revision Books
NCERT Solutions Class 9 Science Chapter 4 Exercise Page: 54
1. Compare the properties of electrons, protons and neutrons.
Solution:
|
Property |
Electrons |
Protons |
Neutrons |
|
Charge |
Negatively charged |
Positively charged |
No charge. |
|
Location |
Located outside the nucleus |
Located within the nucleus |
Located inside the nucleus of an atom |
|
Weight |
Mass is negligible |
1 a.m.u |
1 a.m.u |
|
Affinity |
Attracted towards positively charged |
Attracted towards negatively charged |
Do not get attracted to any charged particle |
2. What are the limitations of J.J.Thomson’s model of the atom?
Solution: The limitations of J.J. Thomson's model of the atom include:
-
Failure to Explain Alpha Particle Scattering: Thomson's model couldn't explain the results of Rutherford's alpha particle scattering experiment. This experiment showed that while most alpha particles passed through the gold foil, some were deflected at large angles and a few even bounced back. Thomson's model, which envisioned electrons embedded in a uniform positive sphere, did not account for the concentrated positive charge and empty space observed in Rutherford's experiment.
-
Lack of Experimental Evidence: Thomson's model was largely theoretical and based on the concept of the "plum pudding" model, where electrons were dispersed within a positively charged sphere. However, it lacked direct experimental evidence to support its claims about the structure and behavior of atoms.
3. What are the limitations of Rutherford’s model of the atom?
Solution: The limitations of Rutherford's model of the atom include:
-
Lack of Expected Stability in Electron Orbits: According to classical electromagnetic theory, an accelerating charged particle (like an electron orbiting a nucleus) should continuously emit energy in the form of electromagnetic radiation. This would cause the electron to lose energy and spiral into the nucleus, leading to instability rather than the observed stable atomic structures.
-
Energy Loss and Atom Stability: The model suggests that atoms, if governed purely by classical physics, would be highly unstable because electrons should continuously lose energy and collapse into the nucleus. However, atoms are observed to be stable entities, which contradicts this prediction
4. Describe Bohr’s model of the atom.
Solution:
-
An atom holds the nucleus at the centre.
-
Negatively charged electrons revolve around the nucleus.
-
The atoms in it contain distinct orbits of electrons.
-
Electrons do not radiate energy when they are in their orbits.
-
The distinct orbits are named K, L, M, and N orbits. Numbers used to denote them are n=1, 2, 3, 4
5. Compare all the proposed models of an atom given in this chapter.
Solution:
|
Thomson |
Rutherford |
Bohr |
|
● Sphere is positively charged. ● Electrons are negatively charged and scattered all through the inside of the sphere. ● Positively charged = negatively charged ● The net charge in the atom is zero. |
● The nucleus is at the centre and is positively charged, holding the entire mass. ● Electrons are negatively charged, revolving in a well-defined path ● In comparison with the nucleus, the size of the atom is very large. ● Force of attraction of the electrons towards the nucleus is balanced by centrifugal force acting away from it. As a result, electrons are not drawn close to the nucleus. |
● Nucleus is present at the centre and is positively charged ● Electrons are negatively charged, revolving around but do not radiate energy. ● The distinct orbits are labelled as K, L, M, and N |
6. Thomson’s Model of Atom.
7. Rutherford’s Model of Atoms.
8. Bohr’s model of the atom.
Summarise the rules for the writing of the distribution of electrons in various shells for the first eighteen elements.
Solution:
-
The maximum number of electrons that can be accommodated in a shell is given by the formula: 2n 2 , where n= 1, 2, 3…
-
The maximum number of electrons in different shells are:
K shell – n=1 ; 2n 2 = 2(1) 2 = 2 L shell – n=2 ; 2n 2 = 2(2) 2 = 8 M shell – n=3 ; 2n 2 = 2(3) 2 = 18 N shell- n=4 ; 2n 2 = 2(4) 2 = 32
-
The outermost orbit can accommodate 8 electrons at the maximum.
-
The electrons are not taken in unless the inner shells are filled, which are filled step-wise; hence, the highest element has K-2; L-8; M-8 distribution of electrons.
9. Define valency by taking examples of silicon and oxygen.
Solution: Valency refers to the combining capacity of an atom, specifically how many electrons an atom needs to gain, lose, or share to achieve a stable electron configuration, typically with a full outer shell (octet rule).
Example of Silicon (Atomic Number 14):
-
Silicon has 14 electrons.
-
Electron distribution: K-shell (2 electrons), L-shell (8 electrons), M-shell (4 electrons).
-
To complete its outermost shell (M-shell), silicon needs 4 more electrons.
-
Therefore, the valency of silicon is 4.
Example of Oxygen (Atomic Number 8):
-
Oxygen has 8 electrons.
-
Electron distribution: K-shell (2 electrons), L-shell (6 electrons).
-
To complete its outermost shell (L-shell), oxygen needs 2 more electrons.
-
Therefore, the valency of oxygen is 2.
10. Explain with examples
(i) Atomic number,
(ii) Mass number,
(iii) Isotopes and
(iv) Isobars.
Give any two uses of isotopes.
Solution: (i) The number of positively charged protons present in the nucleus of an atom is defined as the atomic number and is denoted by Z. Example: Hydrogen has one proton in its nucleus; hence, its atomic number is one. (ii) The total number of protons and neutrons present in the nucleus of an atom is known as the mass number. It is denoted by A. 20 Ca 40 . The mass number is 40. The atomic number is 20. (iii) The atoms which have the same number of protons but a different number of neutrons are referred to as isotopes. Hence, the mass number varies. Example: The most simple example is the Carbon molecule which exists as 6 C 12 and 6 C 14 (iv) Isobars: Isobars are atoms which have the same mass number but differ in atomic number. Examples are, 20 Ca 40 and 18 Ar 40 Uses of isotopes
-
The isotope of the Iodine atom is used to treat goitre, an iodine-deficient disease.
-
In the treatment of cancer, an isotope of cobalt is used.
-
Fuel for nuclear reactors is derived from the isotopes of the Uranium atom.
11. Na+ has completely filled K and L shells. Explain.
Solution: Sodium (Na) has an atomic number of 11, which means it has 11 electrons.
-
Electronic Configuration before Ionization: K-shell (2 electrons), L-shell (8 electrons), M-shell (1 electron)
-
Sodium tends to lose one electron to achieve a stable electron configuration, as elements tend to gain or lose electrons to reach a noble gas configuration.
-
Formation of Sodium Ion (Na^+): When sodium loses one electron, it forms a sodium ion (Na^+).
-
Electronic Configuration after Ionization: K-shell (2 electrons), L-shell (8 electrons).
-
The resulting sodium ion (Na^+) now has a full outer shell (noble gas configuration), making it more stable.
-
It is indeed difficult to remove an electron from a filled shell, as atoms and ions tend to achieve stability by filling or emptying their outermost electron shells.
12. If the bromine atom is available in the form of, say, two isotopes 35 Br 79 (49.7%) and 35 Br 81 (50.3%), calculate the average atomic mass of the Bromine atom.
Solution: The atomic mass of an element is the mass of one atom of that element. Average atomic mass takes into account the isotopic abundance. Isotope of bromine with atomic mass 79 u = 49.7% Therefore, Contribution of 35 Br 79 to atomic mass = (79 × 49.7)/100 ⇒ 39.26 u Isotope of bromine with atomic mass 81 u = 50.3% Contribution of 35 Br 81 to the atomic mass of bromine = (81 × 50.3)/100
⇒ 40.64u
Hence, the average atomic mass of the bromine atom = 39.26 + 40.64 u = 79.9u
13. The average atomic mass of a sample of element X is 16.2 u. What are the percentages of isotopes 8 X 16 and 8 X 18 in the sample?
Solution: Let the percentage of 8 X 16 be ‘a’ and that of 8 X 18 be ‘100-a’. As per the given data, 16.2u = 16 a / 100 + 18 (100-a) /100 1620 = 16a + 1800 – 18a 1620 = 1800 – 2a a = 90% Hence, the percentage of the isotope in the sample 8 X 16 is 90% and that of 8 X 18 = 100-a = 100- 90=10%
14. If Z=3, what would be the valency of the element? Also, name the element.
Solution: Given: Atomic number, Z = 3 The electronic configuration of the element = K-2; L-1, hence its valency = 1 The element with atomic number 3 is Lithium.
15. Composition of the nuclei of two atomic species, X and Y, are given as under
X Y
Protons = 6 6
Neutrons = 6 8
Give the mass numbers of X and Y. What is the relation between the two species?
Solution: Mass number of X: Protons + neutrons = 6+6 = 12 Mass number of Y: Protons + neutrons = 6+8 = 14 They are the same element, and their atomic numbers are the same. They are isotopes, as they differ in the number of neutrons and hence their mass numbers.
16. For the following statements, write T for true and F for false.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons. (b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral. (c) The mass of an electron is about 1/2000 times that of a proton. (d) An isotope of iodine is used for making tincture iodine, which is used as a medicine. Solution: (a) The statement is False. (b) The statement is False. (c) The statement is True. (d) The statement is False.
17. Put a tick(✓) against the correct choice and cross(x) against the wrong choice in questions 15, 16 and 17.
Rutherford’s alpha–particle scattering experiment was responsible for the discovery of
(a) Atomic nucleus (b) Electron (c) Proton (d) Neutron Solution: (a) Atomic nucleus
Isotopes of an element have
(a) The same physical properties (b) Different chemical properties (c) Different number of neutrons (d) Different atomic numbers Solution: (c) Different number of neutrons
18. The number of valence electrons in Cl – ion are
(a) 16 (b) 8 (c) 17 (d) 18 Solution: (b) 8 The electronic distribution of Cl is K-2, L-8, M-7. Valence electrons are 7; hence, chlorine gains one electron for the formation of Cl – . Therefore, its valency is 8.
19. Which one of the following is a correct electronic configuration of Sodium?
(a) 2, 8 (b) 8, 2, 1 (c) 2, 1, 8 (d) 2, 8, 1 Solution: (d) 2, 8, 1
Complete the following table.
|
Atomic Number |
Mass Number |
Number of Neutrons |
Number of Protons |
Number of Electrons |
Name of the Atomic Species |
|
9 16 – – – |
– 32 24 2 1 |
10 – – – 0 |
– – 12 1 1 |
– – – – 0 |
– Sulphur – – – |
Solution: The following table depicts the missing data: Atomic number(Z) = Number of protons Mass number = Number of neutrons + atomic number (or) Mass number(A) = Number of neutrons + number of neutrons
|
Atomic Number |
Mass Number |
Number of Neutrons |
Number of Protons |
Number of Electrons |
Name of the Atomic Species |
|
9 16 12 1 1 |
19 32 24 2 1 |
10 16 12 1 0 |
9 16 12 1 1 |
9 16 12 1 0 |
Fluorine Sulphur Magnesium Deuterium Hydrogen |
Check Out: Class 9 Combo Set of 5 Books
NCERT Solutions for Class 9 Science Chapter 4: Structure of the Atom Benefits
1. Clear Concept Explanations
NCERT Solutions break down complex ideas like atomic models, isotopes, and valency into simple, easy-to-understand language. For example:
-
They explain Rutherford’s gold foil experiment in steps.
-
Define terms like atomic number and mass number with real-world examples (e.g., Carbon-12 vs. Carbon-14).
This clarity helps students grasp fundamentals without confusion.
2. Aligned with CBSE Syllabus
The solutions strictly follow the NCERT textbook, ensuring you study only what’s relevant for exams. Questions like “Calculate the valency of Sulfur” or “Explain Bohr’s model” are answered in the same format expected in CBSE papers.
3. Practice for Exams
The Structure of the Atom Class 9 Questions and Answers section includes:
-
Short answers (e.g., limitations of Rutherford’s model).
-
Numericals (e.g., calculating electrons in shells).
-
Definitions (e.g., isotopes).
Practicing these prepares you for every question type in exams.
4. Builds Strong Foundation
Chapter 4 is the base for higher-class topics like Chemical Bonding and Periodic Table. NCERT Solutions ensure you understand:
-
How electrons determine an element’s reactivity (valency).
-
Why isotopes have the same chemical properties but different physical ones.
5. Improves Problem-Solving Skills
By solving numericals on atomic number and mass number, students learn step-by-step logical thinking. For example:
-
If an atom has 7 protons and 7 neutrons, its mass number = 14.
The journey through Class 9 Science Chapter 4: Structure of the Atom is more than just memorizing protons, neutrons, and electrons; it’s about knowing the building blocks of the universe. This chapter lays the groundwork for advanced topics like chemical reactions, nuclear physics, and even modern technologies like MRI machines and atomic energy.
Check Out: PW School Books
Structure of The Atom FAQS
1. What is an Atom?
An atom is the smallest unit of matter. It has three main particles:
-
Protons: Positively charged.
-
Neutrons: Neutral (no charge).
-
Electrons: Negatively charged.
2. How many Atomic Models are there?
There are three atomics models. They are
-
Thomson’s Model: Atoms are like a “watermelon” with electrons embedded in a positively charged sphere.
-
Rutherford’s Model: Atoms have a tiny, dense nucleus (with protons/neutrons) surrounded by electrons.
-
Bohr’s Model: Electrons move in fixed orbits (shells) around the nucleus.
3. How are isotopes different from isobars?
Isotopes have the same atomic number but different mass numbers (e.g., Carbon-12 and Carbon-14). Isobars have the same mass number but different atomic numbers (e.g., Argon-40 and Calcium-40).
4. How did Thomson’s model differ from Rutherford’s?
Thomson’s model had electrons embedded in a positive sphere, while Rutherford proposed a dense nucleus surrounded by electrons.











