CBSE Class 11 Chemistry Notes Chapter 6 Thermodynamics

Thermodynamics Class 11 is a very important chapter because it explains how energy flows and changes during different processes. In simple words, thermodynamics helps us understand how energy moves from one place to another and how it changes from one form to another. Class 11 Chemistry Chapter 6 Notes explain these ideas clearly for easy learning. Before studying energy changes, the chapter introduces the idea of a system, which is the starting point of any energy exchange.
This chapter is detailed and includes both theory and numerical questions. These questions help students understand how to calculate energy changes in different reactions. To score well, students must strengthen their basics from the beginning. For fast revision, students can download Thermodynamics Class 11 Chemistry Notes PDF from our website for free.
Check Out: Class 11th Books
Thermodynamics Class 11 Notes Overview
The chapter mainly explains energy changes in physical and chemical processes. It covers basic terms like system, surroundings, state of a system, and types of processes.A major portion of Thermodynamics Class 11 Chemistry Notes is the First Law of Thermodynamics, which says that energy cannot be created or destroyed. It can only be converted from one form to another. Students learn concepts like internal energy, heat, work, enthalpy, and heat capacity through simple examples.
The chapter also explains exothermic and endothermic reactions, showing when energy is released or absorbed. Another key topic is the Second Law of Thermodynamics, which introduces entropy. Entropy tells us about the randomness or disorder in a system and helps explain why some reactions happen on their own.
Overall, this Class 11 Chemistry Chapter 6 Notes give students a strong foundation for exams and higher studies in chemistry and physics.
CBSE Class 11 Chemistry Notes Chapter 6
Thermodynamics is the study of the movement of mass, heat, and energy.
Thermodynamics terminology
-
System:
The system is a prominent region of the cosmos that is constantly monitored.
-
Surrounding:
The remaining part of the universe except for the system which isn’t kept under observation is known as surroundings.
In general, it can be stated as;
Universe = System + Surrounding
Types of the system
a) Open system –
The system where the flow of both, mass and heat energy takes place.
Example: Human body.
b) Closed system –
The system where the flow of heat energy takes place but has constant mass.
Example: Pressure cooker.
c) Isolated system –
The system where none of the flow takes place.
Example: Thermos flask.
State of the system
It is possible to define and modify the system's state about modifications in the state variables, P, V, T, and n. Any change in one of these variables will alter the system's status because they determine its circumstances.
Properties of the system
-
Intensive properties –
Properties are mass- and number-independent, depending only on the concentration of the particles in the system. These include density, refractive index, pressure, and so on.
-
Extensive properties –
properties that are dependent on the system's overall particle count or mass. Volume, total energy, etc. are among them.
Thermodynamic equilibrium
When the three types of equilibrium listed below are satisfied and the state variables remain unchanged, the system remains in equilibrium.
-
Mechanical equilibrium –
The absence of mechanical motion, constant pressure, and volume brings up the mechanical equilibrium.
-
Thermal equilibrium –
The constant heat and temperature concerning time bring up thermal equilibrium.
-
Chemical equilibrium –
The rate of forward reaction equal to the rate of backward reaction brings up the chemical equilibrium.
Check out: Class 11th Question Banks
Internal energy
Internal energy, frequently represented by the letters U or E, is the total of the energy's constituent parts that are impacted by the system's internal elements. The system under observation acts as an ideal gas system that depends only upon kinetic energy and hence, is the function of temperature as U ∝ T . Thus, the internal energy is a state function.
Modes of energy transport
Heat
Heat (Q) is the energy that is transmitted as a result of temperature differences in the system and its surroundings. The molecules' kinetic energy increases with system temperature, raising the internal energy as a result.
Work:
Work (W) is the amount of energy used to overcome the external forces operating upon the system. A system's internal energy decreases as it expands. On the other hand, internal energy increases as the system contracts.
The first law of thermodynamics:
The first law of thermodynamics states that energy can neither be created nor destroyed.
The sign conventions are given as;
Work done by the system = - W
Work done on the system = + W
Heat flows into the system = + Q
Heat flows out of the system = - Q
Reversibility
The process can retrace its beginning course and arrive at the same initial state by making a very tiny, or infinitesimal, modification in the system or surroundings. There must be no dissipative forces and the system must be in a quasi-static state for a process to follow reversibility.
-
Quasi-static state –
In this case, the system appears to be static at all times, yet it is not. The system appears to be in balance with its surroundings because of how slowly the motion is occurring.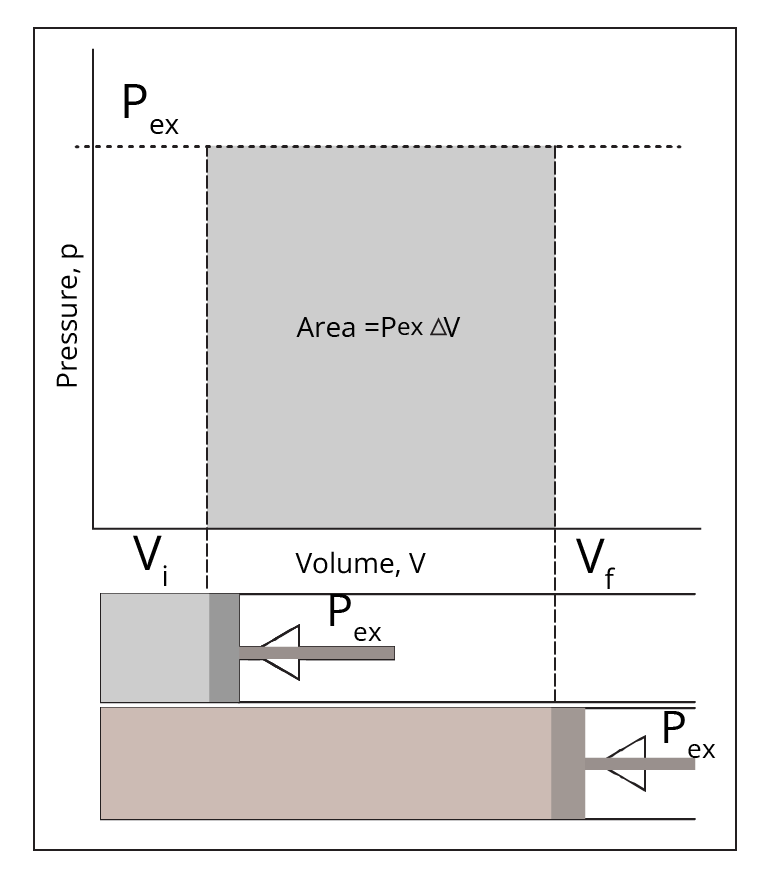
Types of thermodynamic processes
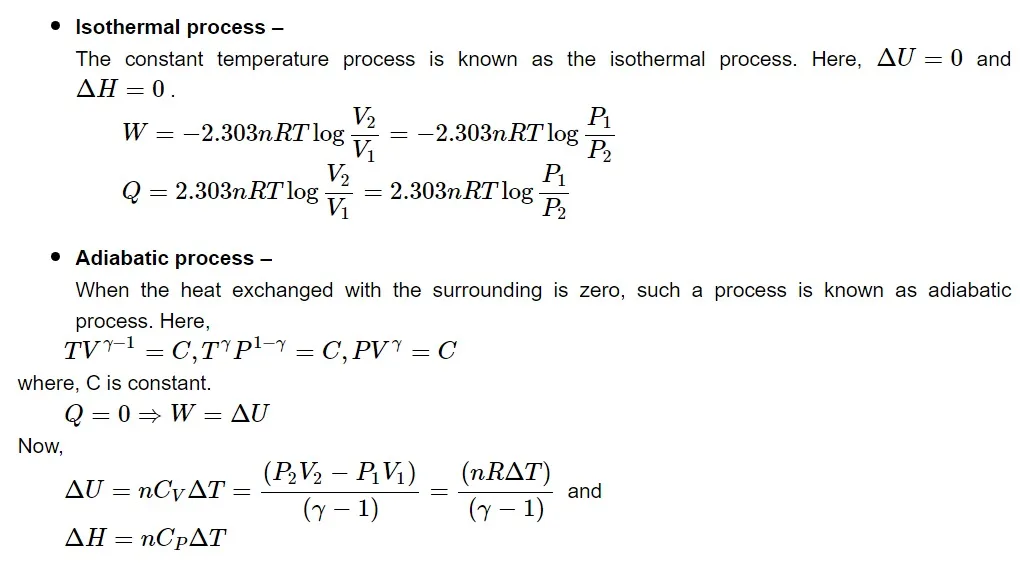
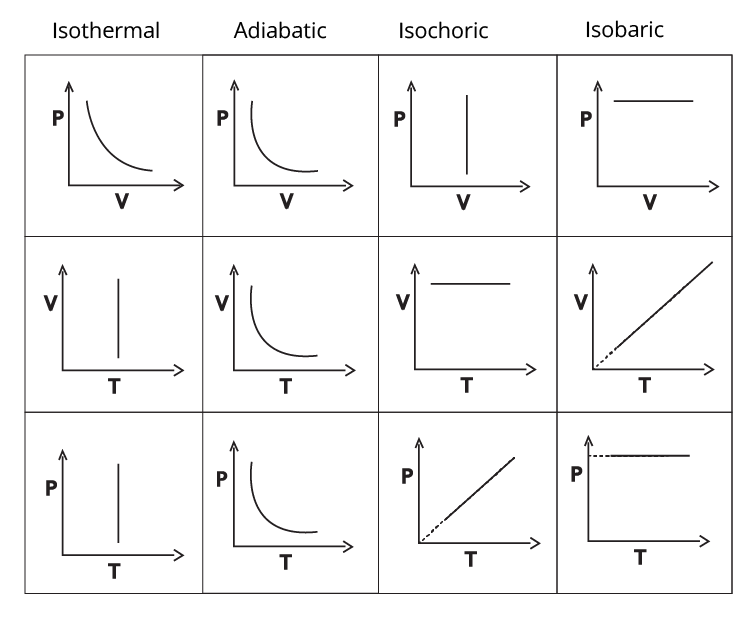
Read More: CBSE Class 11 Syllabus for All Subjects
How to Use Class 11 Chemistry Ch 6 Notes
-
Read NCERT First
Start by reading the complete chapter from NCERT. For example, if you are studying Class 11 Chemistry Chapter Thermodynamics Notes, first understand terms like system, surroundings, energy changes, and laws of thermodynamics. -
Use Notes for Revision
After NCERT, go through Thermodynamics Class 11 Short Notes. These notes simplify the concepts and make revision quick. -
Focus on Key Concepts
Pay attention to important terms, formulas, and definitions such as internal energy, enthalpy, entropy, equilibrium, etc. -
Solve Questions with Notes
Use Class 11 Chemistry Ch 6 Notes while solving NCERT exercises, previous year papers, and question banks. -
Make Short Self-Notes
Write summary notes on topics like the First Law of Thermodynamics or exothermic vs. endothermic processes. This strengthens memory. -
Revise Regularly
Keep revising the notes so concepts stay fresh, especially in subjects that include formulas and diagrams.
Books for Class 11
Using good class 11 books makes your study time more useful. Instead of reading the same chapters again and again, you can test what you actually know. Find the books link on the table
|
Book Name |
Link |
|
CBSE Question Bank Class 11 Chemistry |
|
|
CBSE Question Bank Class 11 Combo Set of 4 Books |
|
|
CBSE Class 11 Chapterwise 20 Most Probable |
|
|
CBSE Class 11 Formula Handbook For 2026 Exams |
CBSE Class 11 Chemistry Notes Chapter 6 FAQs
1. What is thermodynamics in Class 11 Chemistry?
Thermodynamics is the branch of chemistry that studies energy changes during physical and chemical processes. Thermodynamics Class 11 explains how energy is transferred between a system and its surroundings.
2. What is the First Law of Thermodynamics?
It states that energy cannot be created or destroyed; it can only change forms. In any chemical reaction, the total energy of the system remains constant.
3. How should I prepare for Chemistry Class 11 Chapter 6?
Start with NCERT, understand each concept, revise Thermodynamics Class 11 Chemistry Notes, make short notes, and solve previous year papers or question banks.
4. Is NCERT enough for Class 11 Chemistry Thermodynamics?
Yes, NCERT builds strong basics. But for deeper practice, students can also use PYQs, handwritten notes, and Class 11 Chemistry Thermodynamics Notes for revision.










